The trials listed here are being conducted by pharmaceutical companies and physicians who are partnering with or have contacted the Gorlin Syndrome Alliance.
For a complete listing, please go to clinicaltrials.gov.
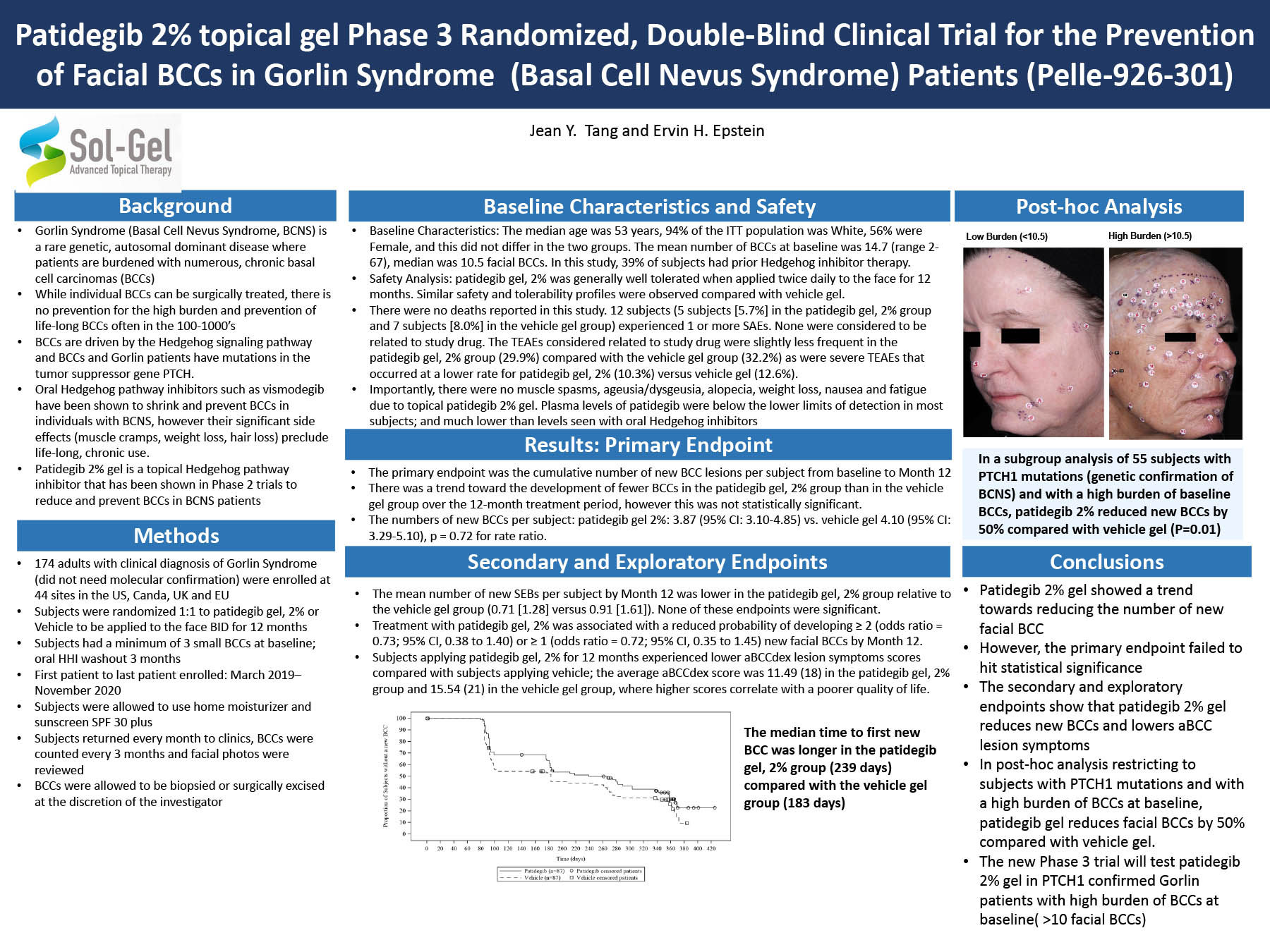
CURRENT CLINICAL TRIAL OPPORTUNITY
“Efficacy and Safety of Patidegib Gel 2% for Preventing Basal Cell Carcinomas on the Face of Adults with Gorlin Syndrome”
TRIAL:
Clinical trial available now for topical gel to prevent basal cell skin cancers (BCCs) in people with Gorlin syndrome.
START DATE:
Ongoing at 30 sites worldwide.
ELIGIBILITY REQUIREMENTS:
These include the following:
- Age 18 or over
- PTCH1 mutation as the drug works best on these people. Free genetic testing will be done at the screening visit if individual meets requirements noted below.
- 10 or more BCCs on your face when you are screened. Specialized, high-powered photography equipment is used to identify the lesions.
- Oral hedgehog inhibitors (Odomzo (sonidegib) or Erivedge (vismodegib)) must be stopped for 3 months prior to first visit.
- Site visits needed: Potential participants will need to go to their chosen clinical trial site for a screening visit. If this is successful, five more in-person site visits will be needed over the course of 12 months. Virtual phone or video visits will also be necessary.
- For more details, please go to: clinicaltrials.gov
LOCATIONS:
30 sites worldwide, over half across the United States. See below for a detailed site list with contact information.
EXPENSES:
All costs associated with your travel will be reimbursed fully. This includes air/bus/train fare, gas and tolls, meals, and overnight lodging as necessary. If a care partner is needed, this individuals’ expenses will be covered as well.
BENEFITS:
- If an individual completes the trial and the medication is FDA approved, this participant will receive the medication FREE for one year.
- With success and FDA approval, we will have a non-surgical, non-invasive preventative therapy for BCCs in the future! YOU will have been a part of creating this opportunity for all adults with Gorlin syndrome.
- Free Genetic Testing (if not previously confirmed) for participants to document PTCH1 mutation.
FOR MORE INFORMATION:
- Please call 717-454-4187 to speak to someone from the GSA about the trial. You can also email us at clinicaltrials@gorlinsyndrome.org
- Information on this trial is also available in a webinar recorded in June of 2024 featuring Dr. Ervin Epstein and Julie Breneiser, Executive Director.
- Visit clinicaltrials.gov
The following clinical trial sites listed below are currently open and actively recruiting.
NORTHEAST
Dr. JaiDe (Jeff) Yu
Location:
Massachusetts General Hospital
Clinical Unit for Research Trials and Outcomes in Skin
50 Staniford Street, Suite 240
Boston, MA 02114
Contact Information –
Trial Coordinator: Anicka Lawrence
Email:
alawrence6@mgh.harvard.edu
Phone numbers
Office: 617-726-5066
Fax: 617-724-2998
Dr. Kristin Bibee
Location:
The Johns Hopkins Hospital
The Johns Hopkins Outpatient Center
601 N. Caroline Street
8th Floor, Suite 8033
Baltimore, MD 21287
Contact Information –
Trial Coordinator:
Manager: Ruizhi Wang
Coordinator: Yage Sun
Email:
rwang@jmmi.edu
yun147@jh.edu
Phone number:
Office: 410-502-7546
Dr. Liz Billingsley
Location:
Penn State Hershey Medical Center
Penn State Health
500 University Drive
Dermatology Research UPC II, Room 2010
Hershey, PA 17033
Contact Information –
Trial Coordinator:
Samantha Gettle
Email:
sgettle2@pennstatehealth.psu.edu
Phone number:
Office: 717-531-4439
Fax: 717-531-5088
Dr. Robert Nossa
Location:
Schweiger Dermatology P.C.
60 Pompton Ave.
Verona, NJ 07044
Contact Information –
Trial Coordinator:
Malgorzata Lasoche Lopez
Email:
malgorzata.lopez@schweigerderm.com
Phone number:
Office: 929-547-0508
Fax: 973-239-8812
SOUTHEAST
Dr. Meenal Kheterpal
Location:
Duke Dermatology
Duke University School of Medicine
40 Duke Medicine Circle
Durham, NC 27710
Contact Information –
Trial coordinator:
Connor Whatley & Kristy Averette
Clinical Research Nurse Coordinator
Email: connor.whatley@duke.edu
Kristy.averette@duke.edu
Phone number:
Office: 919-681-8368
Dr. Cheryl Hull
Location:
Northwest Arkansas Clinical Trials Center, PLLC
599 S. Horsebarn Rd.
Suite 200
Rogers, AR 72758
Contact Information –
Trial coordinator:
Briana Tedford, B.S.
Email: brianahullderm@yahoo.com
Phone numbers
Office: 479-876-8205, Ext. 4
Fax: 479-876-8049
Dr. James Grichnik
Location:
University of South Florida
Department of Dermatology
13330 USF Laurel Drive
Tampa, FL 33612
Contact Information –
Trial coordinator:
Lucy Lam RN, MSMS, CCRC
Email:
llam@usf.edu
Phone number:
Office: 813-974-8249
Dr. Armand Cognetta
Location:
Dermatology Associates of Tallahassee, P.A.
1707 Riggins Road
Tallahassee, FL 32308
Contact Information –
Trial coordinator:
Anna Kaji
Email: akaji@datfi.com
Phone number
Office: 805-728-7931
MIDWEST
Dr. Steve Kempers
Location:
Minnesota Clinical Study Center
119 14th Street NW
Suite 250
New Brighton, MN 48109
Contact Information –
Trial Coordinator:
Jenjira Skrei
Email:
jskrei@associatedskincare.com
Phone number:
Office:
763-571-4200
Dr. Diana Bolotin
Location:
Department of Medicine
Section of Dermatology
5841 S. Maryland Ave.
Chicago, Il 60637
Contact Information –
Trial Coordinator:
Breanna Bertacchi
Email: bbertacchi1@bsd.uchicago.edu
Phone number:
Office: 773-702-7696
Dr. Jose Garcia-Zuazaga
Location #1:
Apex Clinical Research Center
29111 Cedar Road
Mayfield Heights, OH 44124
Contact Information –
Trial coordinator:
Brooke Glivar, LPN, CCRC
Research Site Manager
Email: BGlivar@apexskin.com
Phone numbers:
Cell: 330-933-2180
Office:440-940-2739
Dr. Jose Garcia-Zuazaga
Location #2:
Apex Clinical Research Center
Apex skin Corporate Office
4124 Munson Street
Canton, OH 44718
Contact Information –
Trial coordinator:
Theresa Sedlak-Hanslik, LPN, CCRC
Research Site Manager
Email: BGlivar@apexskin.com
Phone numbers:
Cell: 330-933-2180
Office:440-940-2739
Dr. Melody Stone
Location:
MediSearch Clinical Trials
1427 Village Drive
St. Joseph, MO 64506
Contact Information –
Trial coordinator:
Healthier Jackson
Email:
Heather.Jackson@medisearchderma.com
Phone number:
Office: 816-364-1515
Dr. Mio Nakamura
Location:
University of Michigan Medicine
Department of Dermatology
1618 Taubman Center
1500E. Medical Center Drive
Ann Arbor, MI 48109
Contact Information –
Trial Coordinator:
Nicole Mahn
Email:
dermtrials@med.umich.edu
Phone number:
Office: 734-232-0562
SOUTHWEST
Dr. Timothy Rodgers
Location:
North Texas Center for Clinical Research
3880 Parkwood Blvd, Suite 102
Frisco, TX 75034
Contact Information –
Trial Coordinator:
Ashlyn Vallejo
Email: ashlyn@ntxclinicalresearch.com
Phone numbers:
Cell: 214-618-0220
Office: 940-315-5626
Dr. Lenny Henderson
Location:
SSM Health Dermatology
608 NW 9th Street, Suite 5000
Oklahoma City, OK 73102
Contact Information –
Trial coordinator:
Jessica Lackey
Email: jessica@hightowerclinical.com
Phone number:
Office: 405-579-8331
WEST
Dr. Olivia Lucero
Location:
Oregon Health & Science University
SW Bond Ave
Portland, OR 97239
Contact Information –
Trial coordinator:
Marisa Jingco
Rachel Morimoto
Email: jingco@ohsu.edu
Phone number:
Office: 503-418-9078
Dr. Michelle Aszterbaum
Location:
Dermatology Center of Newport
360 San Miguel Dr Suite 406,
Newport Beach, CA 92660
Contact Information –
Trial coordinator:
Hannah Walton
Email:
hew2018@gmail.com
Phone number:
Cell: 203-564-0030
Dr. Kavita Sarin
Location:
Stanford University School of Medicine – Department of Dermatology
455 Broadway St. Discovery Hall 1st Fl.
Redwood City, CA 94063
Contact Information:
Trial coordinator:
Dom Mitchell
Email:
domcm@stanford.edu
Phone number:
Office: 650-721-7192
Fax: 650-498-5908
Dr. Sunil Dhawan
Location:
Center for Dermatology Clinical Research, Inc.
2557 Mowry Ave., Suite 34
Fremont, CA, USA 94538
Contact Information –
Trial coordinator:
Leanne Saud, MA
Email: leannes@ctr4derm.com
Phone number:
Office: 510-797-0140, Ext. 5
Dr. April Armstrong
Location:
University of California Los Angeles
911 Broxton Ave., 3rd Floor
Los Angeles, CA 90024
Contact Information:
Trial coordinator:
Peggy Chou
Email:
PChou@mednet.ucla.edu
Phone numbers
Cell: 951-256-7538
For more information on this clinical trial, including on inclusion and exclusion criterion, please see the following resources:
- GSA Clinical Trial Informational Webinar featuring Dr. Ervin Epstein Jr. and Julie Breneiser, Executive Director.
- Visit clinicaltrials.gov
Questions about Gorlin syndrome that are not related to this clinical trial should be directed to info@gorlinsyndrome.org or call 267-689-6443
Further information on this trial will be provided as soon as it becomes available.
Please consider becoming a hero to the Gorlin syndrome community by participating in this clinical trial.
Thank You