The trials listed here are being conducted by pharmaceutical companies and physicians who are partnering with or have contacted the Gorlin Syndrome Alliance.
For a complete listing, please go to clinicaltrials.gov.
CURRENT CLINICAL TRIAL OPPORTUNITIES
Blue-Light Photodynamic Therapy + Sonidegib for Basal Cell Carcinomas
Study Title: Blue-Light Photodynamic Therapy and Sonidegib for Multiple Basal Cell Carcinomas
Location: Phoenix, Arizona, United States
ClinicalTrials.gov Identifier: NCT06623201
Summary
This research study is testing combination Blue-light photodynamic therapy and Sonidegib as a possible treatment for people with multiple basal cell carcinoma lesions. Photodynamic therapy uses light along with a drug applied to the skin to kill the cancer cells and cause them to break apart. The light used can cause the skin to feel warm, but does not cause scarring.
Key Details
Condition Studied: Basal Cell Carcinoma (multiple lesions)
Study Type / Phase: Interventional, Phase 1
Recruitment Status: Recruiting
Interventions:
Topical application of aminolevulinic acid (ALA, Levulan) plus PDT using the BLU-U device (blue light illumination)
Sonidegib 200 mg orally daily for 3 months as part of the treatment regimen
Ages Eligible for Study: ≥ 18 years
Gender Eligibility: All genders
Location / Site: Medical Dermatology Specialists, Phoenix, AZ
Principal Investigator: Nathalie Zeitouni, MD, Medical Dermatology Specialists
Contact: Sarah Berman, Medical Dermatology Specialists, (Phone: 602-354-5770 / Email: saberman@usdermpartners.com)
Participants who meet eligibility criteria at baseline will receive Sonidegib 200 mg by mouth every day for 3 months. Participants will undergo three PDT sessions with topical application of ALA at Day 7, Day 30, and Day 90.
The drug applied to the skin before the light treatment is an FDA approved drug called Levulan and has no known side effects.
Eligibility Criteria
Patients will be eligible for inclusion if they meet these study criteria in addition to the others listed on the clinicaltrials.gov website NCT06623201:
- Male or females, at least 18 years of age
2. Diagnosis of BCC with at least 3 nodular lesions that measure 0.5 cm to 5 cm in diameter, located on the head and neck, trunk or extremities.
3. Diagnosis must be confirmed clinically at baseline with 1-2 lesions having been biopsied no sooner than 2 weeks prior to treatment.
4. Patients who may have high burden of disease ie large lesions, who are non-surgical candidates or who refuse surgery.
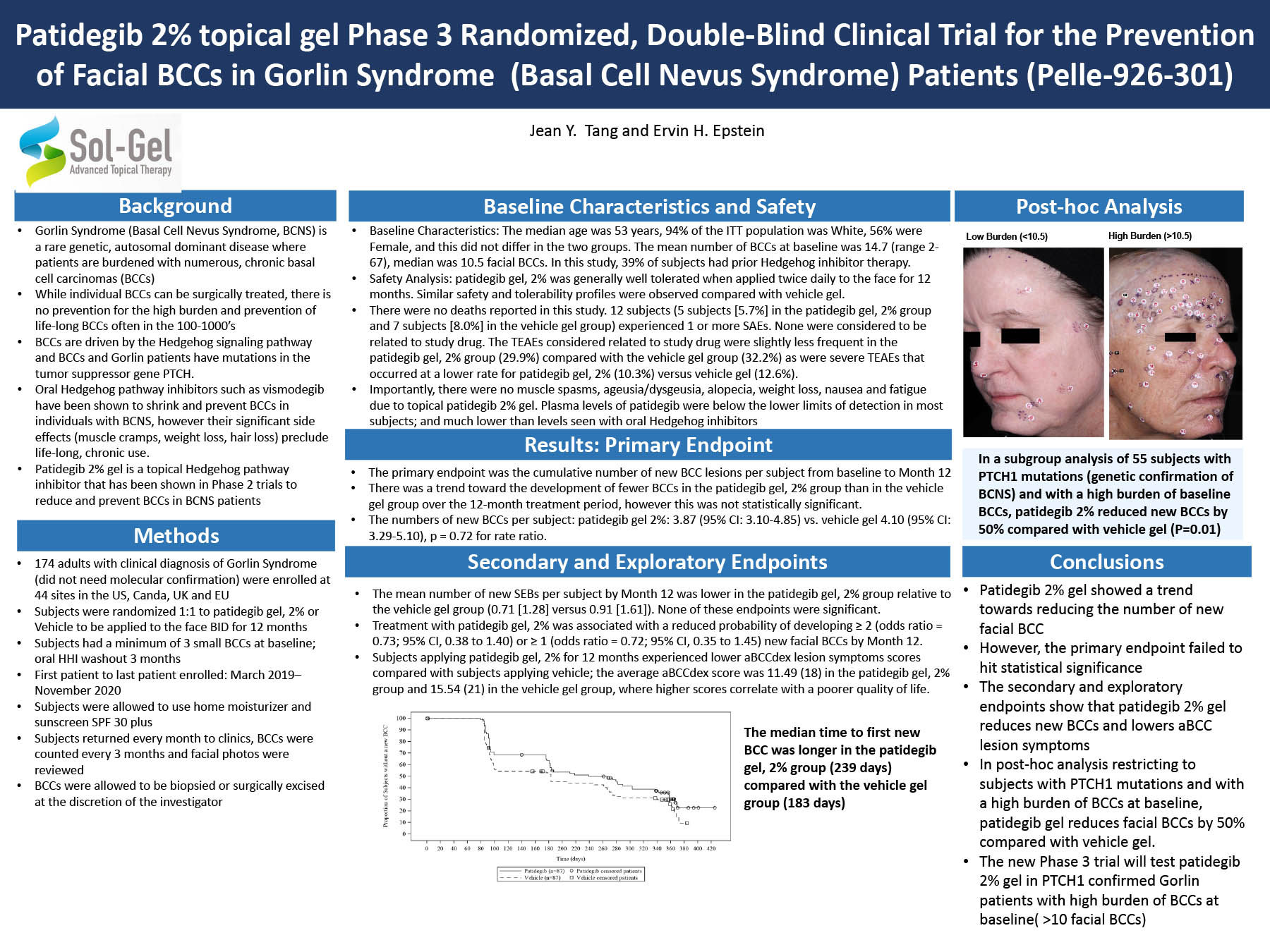
“Efficacy and Safety of Patidegib Gel 2% for Preventing Basal Cell Carcinomas on the Face of Adults with Gorlin Syndrome”
TRIAL:
Clinical trial available now for topical gel to prevent basal cell skin cancers (BCCs) in people with Gorlin syndrome.
START DATE:
Ongoing at 43 sites worldwide.
ELIGIBILITY REQUIREMENTS:
These include the following:
- Age 18 or over
- PTCH1 mutation as the drug works best on these people. Free genetic testing will be done at the screening visit if individual meets requirements noted below.
- 10 or more BCCs on your face when you are screened. Specialized, high-powered photography equipment is used to identify the lesions.
- Oral hedgehog inhibitors (Odomzo (sonidegib) or Erivedge (vismodegib)) must be stopped for 3 months prior to first visit.
- Site visits needed: Potential participants will need to go to their chosen clinical trial site for a screening visit. If this is successful, five more in-person site visits will be needed over the course of 12 months. Virtual phone or video visits will also be necessary.
- For more details, please go to: clinicaltrials.gov
LOCATIONS:
43 sites worldwide, over half across the United States. See below for a detailed site list with contact information.
EXPENSES:
All costs associated with your travel will be reimbursed fully. This includes air/bus/train fare, gas and tolls, meals, and overnight lodging as necessary. If a care partner is needed, this individuals’ expenses will be covered as well.
The following compensation will be offered at all trial locations.
- $500 per each on-site visit
- $350 per each remote visit
- $750 at last visit upon completion of all obligations such as diary, questionnaires, etc.
If you refer family and friends with Gorlin syndrome, you may be eligible for further financial incentives as well.
BENEFITS:
- If an individual completes the trial and the medication is FDA approved, this participant will receive the medication FREE for one year.
- With success and FDA approval, we will have a non-surgical, non-invasive preventative therapy for BCCs in the future! YOU will have been a part of creating this opportunity for all adults with Gorlin syndrome.
- Free Genetic Testing (if not previously confirmed) for participants to document PTCH1 mutation.
FOR MORE INFORMATION:
- Information on this trial is also available in a webinar recorded in June of 2024 featuring Dr. Ervin Epstein and Julie Breneiser, Executive Director.
- Visit clinicaltrials.gov
The following clinical trial sites listed below are currently open and actively recruiting.
NORTHEAST
Dr. Kristin Bibee
Location:
The Johns Hopkins Hospital
The Johns Hopkins Outpatient Center
601 N. Caroline Street
8th Floor, Suite 8033
Baltimore, MD 21287
Contact Information –
Trial Coordinator:
Manager: Ruizhi Wang
Coordinator: Yage Sun
Email:
rwang@jmmi.edu
yun147@jh.edu
Phone number:
Office: 410-502-7546
Dr. Liz Billingsley
Location:
Penn State Hershey Medical Center
Penn State Health
500 University Drive
Dermatology Research UPC II, Room 2010
Hershey, PA 17033
Contact Information –
Trial Coordinator:
Samantha Gettle
Email:
sgettle2@pennstatehealth.psu.edu
Phone number:
Office: 717-531-4439
Fax: 717-531-5088
Dr. Robert Nossa
Location:
Schweiger Dermatology P.C.
60 Pompton Ave.
Verona, NJ 07044
Contact Information –
Trial Coordinator:
Malgorzata Lasoche Lopez
Email:
malgorzata.lopez@schweigerderm.com
Phone number:
Office: 929-547-0508
Fax: 973-239-8812
Dr. Sean Christensen
Location:
Yale University School of Medicine
Yale Center for Clinical Investigation
2 Church Street South
Suite 115
New Haven, CT 06519
Contact Information –
PI: Dr. Sean Christensen
Study Coordinator: Nicole Olszewski
Email: nicole.olszewski@yale.edu
Phone: 203-785-5505
Dr. Anthony Rossi
Location:
Memorial Sloan Kettering Cancer Center
1275 York Ave
New York, NY 10065
Contact Information –
PI: Dr. Anthony Rossi
Study Coordinator: Shanti Persaud
Contact: Patient Referral Service – United States: 984-275-0103
Contact: Study Coordinator
646-608-2311 Email: mangarl@mskcc.org
SOUTHEAST
Dr. Meenal Kheterpal
Location:
Duke Dermatology
Duke University School of Medicine
40 Duke Medicine Circle
Durham, NC 27710
Contact Information –
Trial coordinator:
Connor Whatley & Kristy Averette
Clinical Research Nurse Coordinator
Email: connor.whatley@duke.edu
Kristy.averette@duke.edu
Phone number:
Office: 919-681-8368
Dr. Cheryl Hull
Location:
Northwest Arkansas Clinical Trials Center, PLLC
599 S. Horsebarn Rd.
Suite 200
Rogers, AR 72758
Contact Information –
Trial coordinator:
Briana Tedford, B.S.
Email: brianahullderm@yahoo.com
Phone numbers
Office: 479-876-8205, Ext. 4
Fax: 479-876-8049
Dr. James Grichnik
Location:
University of South Florida
Department of Dermatology
13330 USF Laurel Drive
Tampa, FL 33612
Contact Information –
Trial coordinator:
Lucy Lam RN, MSMS, CCRC
Email:
llam@usf.edu
Phone number:
Office: 813-974-8249
Dr. Armand Cognetta
Location:
Dermatology Associates of Tallahassee, P.A.
1707 Riggins Road
Tallahassee, FL 32308
Contact Information –
Trial coordinator:
Anna Kaji
Email: akaji@datfi.com
Phone number
Office: 805-728-7931
MIDWEST
Dr. Steve Kempers
Location:
Minnesota Clinical Study Center
119 14th Street NW
Suite 250
New Brighton, MN 48109
Contact Information –
Trial Coordinator:
Jenjira Skrei
Email:
jskrei@associatedskincare.com
Phone number:
Office:
763-571-4200
Dr. Jose Garcia-Zuazaga
Location #1:
Apex Clinical Research Center
29111 Cedar Road
Mayfield Heights, OH 44124
Contact Information –
Trial coordinator:
Brooke Glivar, LPN, CCRC
Research Site Manager
Email: BGlivar@apexskin.com
Phone numbers:
Cell: 330-933-2180
Office:440-940-2739
Dr. Jose Garcia-Zuazaga
Location #2:
Apex Clinical Research Center
Apex skin Corporate Office
4124 Munson Street
Canton, OH 44718
Contact Information –
Trial coordinator:
Theresa Sedlak-Hanslik, LPN, CCRC
Research Site Manager
Email: BGlivar@apexskin.com
Phone numbers:
Cell: 330-933-2180
Office:440-940-2739
Dr. Melody Stone
Location:
MediSearch Clinical Trials
1427 Village Drive
St. Joseph, MO 64506
Contact Information –
Trial coordinator:
Healthier Jackson
Email:
Heather.Jackson@medisearchderma.com
Phone number:
Office: 816-364-1515
Dr. Mio Nakamura
Location:
University of Michigan Medicine
Department of Dermatology
1618 Taubman Center
1500E. Medical Center Drive
Ann Arbor, MI 48109
Contact Information –
Trial Coordinator:
Nicole Mahn
Email:
dermtrials@med.umich.edu
Phone number:
Office: 734-232-0562
Dr. Christine Poblete-Lopez
Location:
Cleveland Clinic – Main Campus
9500 Euclid Ave
Cleveland, OH 44195
Contact Information –
PI: Dr. Christine Poblete-Lopez
Study Coordinator: Beverly Doyle
Email: doyleb4@ccf.org
Phone number: 216-636-1196
SOUTHWEST
Dr. Timothy Rodgers
Location:
North Texas Center for Clinical Research
3880 Parkwood Blvd, Suite 102
Frisco, TX 75034
Contact Information –
Trial Coordinator:
Ashlyn Vallejo
Email: ashlyn@ntxclinicalresearch.com
Phone numbers:
Cell: 214-618-0220
Office: 940-315-5626
Dr. Lenny Henderson
Location:
SSM Health Dermatology
608 NW 9th Street, Suite 5000
Oklahoma City, OK 73102
Contact Information –
Trial coordinator:
Jessica Lackey
Email: jessica@hightowerclinical.com
Phone number:
Office: 405-579-8331
Dr. Michael Migden
Location:
The University of Texas MD Anderson Cancer Center
1515 Holcombe Blvd
Houston, TX 77030
Contact Information –
PI: Dr. Michael Migden
Study Coordinator: Nitzia Quilantan Barragan
Email: NEQuilantan@mdanderson.org
Phone: 713-834-7768
WEST
Dr. Olivia Lucero
Location:
Oregon Health & Science University
SW Bond Ave
Portland, OR 97239
Contact Information –
Trial coordinator:
Marisa Jingco
Rachel Morimoto
Email: jingco@ohsu.edu
Phone number:
Office: 503-418-9078
Dr. Michelle Aszterbaum
Location:
Dermatology Center of Newport
360 San Miguel Dr Suite 406,
Newport Beach, CA 92660
Contact Information –
Trial coordinator:
Hannah Walton
Email:
hew2018@gmail.com
Phone number:
Cell: 203-564-0030
Dr. Kavita Sarin
Location:
Stanford University School of Medicine – Department of Dermatology
455 Broadway St. Discovery Hall 1st Fl.
Redwood City, CA 94063
Contact Information:
Trial coordinator:
Dom Mitchell
Email:
domcm@stanford.edu
Phone number:
Office: 650-721-7192
Fax: 650-498-5908
Dr. Sunil Dhawan
Location:
Center for Dermatology Clinical Research, Inc.
2557 Mowry Ave., Suite 34
Fremont, CA, USA 94538
Contact Information –
Trial coordinator:
Leanne Saud, MA
Email: leannes@ctr4derm.com
Phone number:
Office: 510-797-0140, Ext. 5
Dr. April Armstrong
Location:
University of California Los Angeles
911 Broxton Ave., 3rd Floor
Los Angeles, CA 90024
Contact Information:
Trial coordinator:
Peggy Chou
Email:
PChou@mednet.ucla.edu
Phone numbers
Cell: 951-256-7538
For more information on this clinical trial, including on inclusion and exclusion criterion, please see the following resources:
- GSA Clinical Trial Informational Webinar featuring Dr. Ervin Epstein Jr. and Julie Breneiser, Executive Director.
- Visit clinicaltrials.gov
Questions about Gorlin syndrome that are not related to this clinical trial should be directed to info@gorlinsyndrome.org or call 267-689-6443
Further information on this trial will be provided as soon as it becomes available.
Please consider becoming a hero to the Gorlin syndrome community by participating in this clinical trial.
Thank You